The Covid19 vaccine has received emergency approval in countries such as the USA and UK, and we aren’t too far off having the vaccination safely approved by the TGA for use in Australia.
We have put together a fact sheet of some FAQ about the vaccines for you to read. If you have any further enquiries please get in contact with us and we will get back to you.
What vaccinations will be available in Australia?
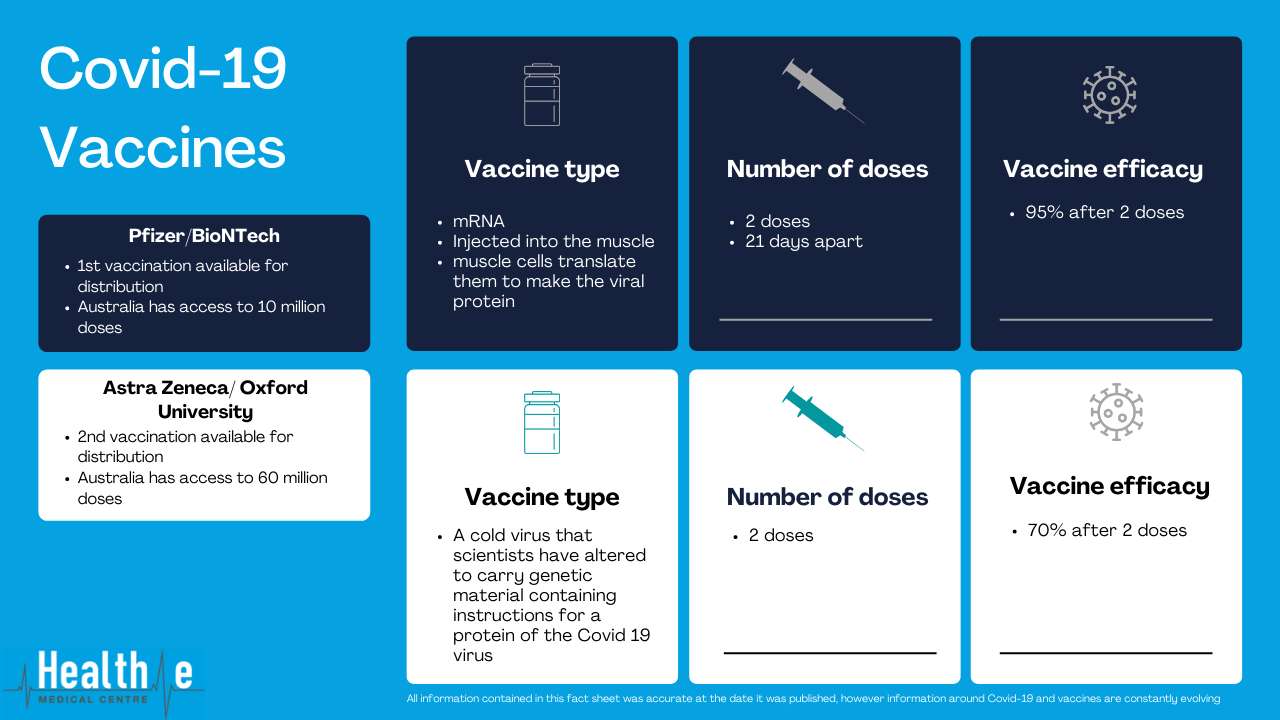
The first vaccination that will be available for use is the Pfizer/BioNTech vaccination. This vaccination will be rolled out to the first priority groups – aged care residents and workers, frontline health workers, and border/quarantine workers.
The Pfizer/BioNTech vaccination has a 95% efficacy and the two injections (given 21 days apart). It uses mRNA technology, meaning the vaccine carriers the genetic material that encodes a portion of Covid19. When these genetic instructions are injected into the muscle, the muscle cells translate them to make the viral protein directly in the body, giving the immune system a preview of the virus without causing the disease. The body can then build the antibodies against the Covid19 disease.
The second vaccination that Australia is likely to receive is the AstraZeneca / Oxford Universityvaccination. This is also a two dose vaccination, and is said to be around 70% effective according to preliminary data (this may change as more data becomes available). This vaccination uses a cold virus that scientists have tweaked to carry genetic material containing instructions for a protein of the covid19 virus. When injected, our bodies produce the coronavirus protein, triggering an immune response.
When will the vaccination be available?
The Australian government has advised that the Pfizer/BioNTech vaccination will be available from mid to late February for the first priority groups. This is dependent on a number of important factors, most importantly, final TGA approval and the delivery of the vaccine from our suppliers.
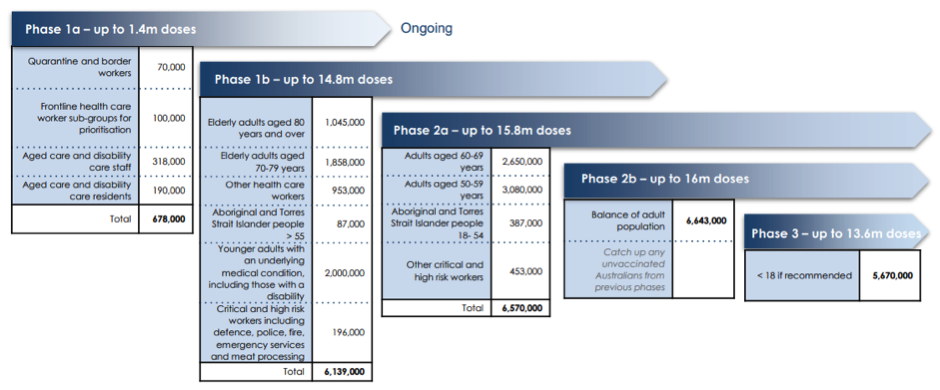
Who gets the vaccination first?
The Australian government has developed a roadmap for the roll-out of the vaccination.
Is the vaccination safe?
Short answer – Yes! Before a COVID-19 vaccine is approved for use in Australia, it must pass the Therapeutic Goods Administration’s (TGA) rigorous assessment and approval processes. This includes assessment of its safety, quality and effectiveness.
How was the vaccination made so quickly?
Pharmaceutical companies often face a number of challenged when developing a new vaccine. This includes funding, trial participants, bureaucracy and prevalence of disease. With the Covid19 pandemic affecting so many people, with such a huge mortality and morbidity rate, researchers had enough funding and support to focus all of their efforts on development of the vaccine, and then were able to stream line their processes to be able to move through the trial phases more efficiently.
Are there any side effects?
The vaccinations have undergone a full and comprehensive trial study with tens of thousands of people, and has been administered now to millions more. Common reported side effects include:
- Localised reaction at injection site (pain/redness/swelling)
- Fever
- Fatigue
- Headache
- Muscle & joint pain
Has there been enough time to test for long term safety?
The majority of vaccine reactions occur within the first few hours of vaccination. The rate of severe reactions are roughly 1 in a million. Covid19 has shown to acutely affect the lungs, heart and sometimes the brain. It also has chronic effects for a number of people – much more than those affected by vaccine side effects.
Whilst there hasn’t been enough time to study long-term effects, researchers do have a solid grasp on human physiology and how the vaccine interacts with the immune system at different doses. There are also stringent monitoring protocols in place.